AI-powered digital twins set to revolutionize bone metastasis research
(Notizia in italiano nell'articolo correlato di seguito) University of Pavia contributes to breakthrough study on digital twins of bone metastasis published in «Cancer Research».
An international team including University of Pavia, Politecnico di Milano, La Rochelle University (FR), Houston Methodist Research Institute (USA), and MD Anderson Cancer Center (USA) has achieved a major milestone in oncology research, with a study just published in «Cancer Research».
The work introduces A(BM)², the first in vivo-inspired digital twins of bone metastasis, capable of reproducing how prostate and kidney cancers colonize bone, interact with its microenvironment, and respond to therapies
These advanced agent-based computational models harness artificial intelligence techniques to integrate biological imaging, experimental data, and mathematical simulations. AI-driven calibration ensured that the digital twins realistically captured tumor cell growth, angiogenesis (blood vessel formation), and osteolysis (bone degradation driven by tumor–osteoclast interactions). This allowed the models to act as virtual patients, where complex biological processes can be studied and therapies tested with unprecedented detail.
The study demonstrated that the digital twins could accurately replicate the effects of drugs such as cabozantinib (targeting tumor vasculature) and zoledronic acid (limiting bone resorption). Beyond validating current treatments, the models offer predictive power for novel therapeutic strategies, highlighting how AI and computational modeling can dissect the biological “ecosystem” of metastasis—from tumor–bone interactions to vascular support and therapy resistance.
Professor Pietro Cerveri’s group at the University of Pavia contributed through expertise in mathematical modeling and biomedical image processing, supporting the development of the computational framework and data integration strategies that made the digital twin models possible.
The potential impact of the overall contribution is transformative: by reducing reliance on animal models, accelerating drug development, and opening the way to personalized treatment simulations, this research underscores how artificial intelligence, biomedical engineering, and cancer biology can converge to reshape the future of oncology. This achievement highlights how biomedical engineering and computational science at University of Pavia are driving innovation at the cutting edge of cancer research.
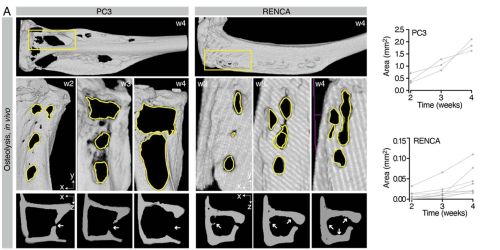
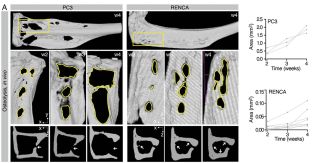
The images above showcase how the progression of osteolysis is reproduced both in in-vivo experiments and in the A(BM)² simulation. In mice, implantation of tumor cells (PC3 or RENCA) leads to progressive bone loss, measured by µCT, with PC3 cells being more osteolytic than RENCA cells.
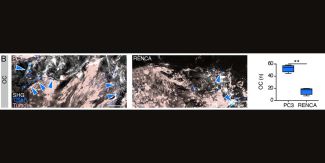
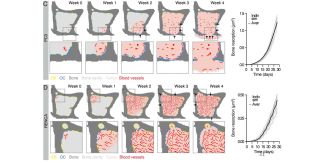
Immunofluorescence analysis confirms that this process is associated with increased recruitment of osteoclasts (OC) by PC3. The same behavior is then recreated in the computational model, where osteoclasts are activated when tumors are located near the bone surface, leading to tissue resorption and replacement with tumor cells.
In summary, the figure demonstrates that A(BM)² is capable of consistently reproducing the tumor-specific differences observed in vivo in the process of osteolysis, highlighting the importance of tumor type and its proximity to bone in determining the severity of bone destruction.
Potrebbe interessarti anche